

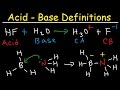














1) Identify the acid, base, conjugate acid, and conjugate base of OCl + H2O -----> HOCl + OH Acid: H2O Base: OCl Conjugate acid: HOCl Conjugate Base: OH Got the wrong answer? Click Here to review Click Here to go back to the QUIZ!! 2) Identify the acid, base, conjugate acid, and conjugate base of HF + H20 -----> F + H3O Acid: HF Base: H2O For the conjugate base, simply remove a proton from the parent molecule/species and CONSERVE mass and charge. If I remove H^+ from H_2O, clearly I get the hydroxide ion, OH^-. Mass and charge have been conserved. If I remove a proton from sulfuric acid, I get bisulfate anion, HSO_4^-; thus bisulfate is the conjugate base of sulfuric acid. Question: What Are The Conjugate Acid-base Pairs In The Reaction Of H2SeO4 With H2O? H2SeO4+H2O→products H2SeO4+H2O→products This problem has been solved! What are conjugate acid base pair? Conjugate Acid- Base pair are the acid base pair which differ only by H+ (Proton). Acids after losing proton forms corresponding conjugate bases . Bases after gaining proton forms corresponding conjugate acid. E.g. (H2O and H3O+) , (H2O AND OH-) , (NH3, NH2–) , (NH3, NH4+). Simple right? H20 → H+ + OH-. Its conjugate base therefore is OH-. The interesting thing here is. The reactant H20 acts like both an acid and a base and produces both a conjugate acid (H+) and a conjugate base (OH-). So actually under standard conditions H20 produces an equal amount of both. maybe you’ve seen this Pkw = 14. Remove a proton to make a conjugate base. In water H 2O adding a proton gives you the hydronium ionH 3O. Answer link. anor277. Nov 26, 2015. The conjugate acid is simply the original species PLUS a proton, H +. The conjugate base is simply the original species LESS a proton, H +. The conjugate base of H 2 O is OH-1, which is the polyatomic ion hydroxide. When we remove a hydrogen ion (proton) from a conjugate acid, the result... See full answer below. Let us solve the problem by taking an example as shown below: HA (aq) + HOH(l) -- H3O+(aq) + A- (aq)? Here,HA loses the H+ to become A-. So HA is acid and A- the conjugate base. Which of the following is the conjugate base of HPO42-? a. H2O b. H3PO4 c. H2PO4- d. PO43- e. P2O5? Answer Save. 2 Answers. Relevance. Dr.A. Lv 7. 1 decade ago. Favorite Answer. HPO42- + H2O = PO43- + H3O+ HPO42- = acid / PO43- = conjugate base. 4 6. Abigail. 4 years ago. D. PO4^3-0 0. Still have questions? Get your answers by asking now. Ask The conjugate base for H2O is the hydroxide ion, OH-. When the hydroxide ion reacts with another water molecule, a hydrogen ion may be transferred, resulting in a water molecule and a hydroxide ion.
[index] [2659] [4903] [2861] [516] [7324] [3524] [2115] [1796] [8531] [4259]
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... This chemistry video tutorial explains the concept of acids and bases using the arrhenius definition, bronsted - lowry and lewis acid base definition. It al... Chemistry: Learn more at: http://www.pathwaystochemistry.com/chemistry-qa/videos/conjugate-acid-base-pairs/ The content of this video is designed to accompany the 12th edition of "Chemistry The Central Science" by Brown, Lemay, Bursten, Murphy, and Woodward. The ti... +2 pts per boxconjugate base stabilization increases acid strength Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺)....
Copyright © 2024 best.nivellesports.site